palladium electron configuration|Electron configuration of palladium : iloilo Palladium Electron Configuration: Electron configuration is conducted having a chemical element in which the distribution of the electrons for that particular chemical item is made for the orbital or the molecular. It helps in .
coolpad y83-100 hard reset,How to, How to hard reset, how to reset, how to hard reset coolpad mega, reset coolpad mega, coolpad mega factory settings, how to.
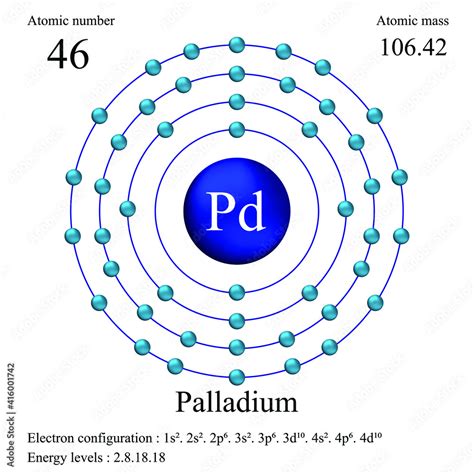
palladium electron configuration,Mar 23, 2023 Learn the electron configuration of palladium, a transition metal in group 10 of the periodic table, with 46 electrons and [Kr]4d10 configuration. Find out its characteristics, obtaining, abundance .

Learn how to write the electron configuration of palladium using the Aufbau principle triangle and the full d orbitals. See the explanation and the answer link for more .Learn how to write the electron configuration of palladium, a silvery white metal with 10 valence electrons. Explore its atomic properties, orbitals, and applications in catalysis and hydrogen . Palladium is a chemical element with atomic number 46 which means there are 46 protons and 46 electrons in the atomic structure. The chemical symbol for Palladium is Pd. . Palladium Electron Configuration: Electron configuration is conducted having a chemical element in which the distribution of the electrons for that particular chemical item is made for the orbital or the molecular. It helps in .Palladium is a shiny, silvery-white metal that resists corrosion and has the electron configuration [Kr] 4d 10. It is used in catalytic converters, jewellery, ceramic capacitors and hydrogenation .
Palladium is a transition metal with symbol Pd and atomic number 46. Its electron configuration is [Kr] 4d 10, which means it has 10 valence electrons and 4 shells.Physical and chemical properties of Palladium: general data, thermal properties, ionization energies, isotopes, reduction potentials, abundance of elements, crystallographic data.

The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron .The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing .The order of filling the orbitals with electrons in the Pd atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 8 But in reality, two electrons move from the 5s orbital to the 4d orbital: Electronic configuration of the Palladium atom in ascending order of orbital energies:palladium electron configuration Electron configuration of palladium Palladium atoms have 46 electrons and the shell structure is 2.8.18.18.0. The ground state electron configuration of ground state gaseous neutral palladium is [ Kr ]. 4d 10 and the term symbol is 1 S 0 .
palladium electron configurationElectronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Palladium (Pd): Palladium is a d-block element having atomic number 46. The group number and period number of Palladium are 10 and 5 respectively. The electron configuration is drawn as follows using the octet rule, where the first two electrons were filled followed by 8 and 18 and the remaining 18 were filled in the last orbital which yields 2, 8, 18, and 18 configurations.. Electronic configuration of Pd Palladium electron configuration notation. The electronic configuration notation of Pd is written as .The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.
Palladium atoms have 46 electrons and the shell structure is 2.8.18.18.0. The ground state electron configuration of ground state gaseous neutral palladium is [ Kr ]. 4d 10 and the term symbol is 1 S 0 .
Electron configuration of palladiumPalladium, complete electron configuration. © 2009-2016 | www.prvky.com | kontaktkontaktLe Palladium a une configuration Electron qui s'écrit comme suit : 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10, c'est la version complète. La configuration électronique simplifiée ou abrégée du palladium est [Kr]4d10. Electron Configuration and Oxidation States of Palladium. Electron configuration of Palladium is [Kr] 4d10. Possible oxidation states are +2,4. Electron Configuration. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties.
Example of Determining Energy Levels (n) For example, if we want to determine the electron configuration for Cobalt (Co) at ground state, we would first look at the row number, which is 4 according to the periodic table below; meaning n = 4 for the s-orbital.In addition, since we know that the energy level for the d orbital is "n-1", therefore n = 3 for the d-orbital in this case.
Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at .
The electron configuration tells us about the distribution of electrons of any molecule in its molecular orbits. The electron configuration of palladium is expressed as $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}4{p^6}5{s^0}4{d^{10}}$. It can also be written as:$[Kr]4{d^{10}}$ Properties of palladium: The Palladium element has the 46 electrons you can refer to the periodic table to calculate that and we further know about the S orbital, which can only retain the maximum 2 number of electrons. Further P can hold 6, d can hold 10 and the f can at last hold 14. With this equation the electron configuration becomes as 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. .Palladium 有一个 Electron 配置,写成如下:1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10,这是完整的版本。 钯的简化或缩写电子构型是 [Kr]4d10。
palladium electron configuration|Electron configuration of palladium
PH0 · What is the electron configuration of palladium?
PH1 · Palladium (Pd)
PH2 · Palladium
PH3 · How To Find The Electron Configuration For
PH4 · Electron configuration of palladium
PH5 · Electron Configuration For Palladium
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Complete Electron Configuration for Palladium (Pd, Pd2+)